USP Chapters 795 and 797
Updated United States Pharmacopeia (USP) Chapters 795 and 797 are now in effect as of November 1, 2023. Covetrus® Compounding Pharmacies is USP compliant, and we are here to help support you in navigating these changes.

What is USP?
- United States Pharmacopeia
- An independent, scientific organization that develops and disseminates public quality standards for medicines and other articles
- USP standards are recognized in law and play a prominent role in federal state compliance and enforcement activities
Which drugs are required to adhere to USP standards?
- Any drug that is recognized in the USP–NF must adhere to USP standards for identity, strength, quality, purity, packaging, and labeling or risk being deemed adulterated or misbranded under state and/or federal pharmacy laws
- These provisions do not differentiate between branded and compounded medications
What are USP Chapters 795 and 797?
- Updated guidelines specifying new default beyond-use dates (BUDs) of compounded products
- USP 795 addresses non-sterile products while USP 797 addresses sterile products
What has changed?
- Water containing products, with activity factor greater than 0.6, have shorter dating (BUD)
- The quantity of compounded medication prescribed will likely need to be decreased and more refills added
- There is a reduced offering of flavors in accordance with these chapters
- Liquids do not have one default BUD since there are many different types (preserved, non-preserved, oil based, water based, etc.)
Accessing BUD in Non-Sterile Compounded Products
TYPES OF PREPARATION | PREVIOUS USP <795> BUD (DAYS) | UPDATED USP <795> BUD (DAYS) | STORAGE TEMPERATURE |
AQUEOUS DOSAGE FORMS | |||
Non-preserved aqueous dosage forms | 14 days | 14 days | Refrigerated |
Preserved aqueous dosage forms | 14 days | 35 days | Controlled room temperature or refrigerated |
NONAQUEOUS DOSAGE FORMS | |||
Oil-based liquids | 180 days | 90 days | Controlled room temperature or refrigerated |
Tablets, capsules, powders | 180 days | 180 days | Controlled room temperature or refrigerated |
BUD Limit by Type of Preparation in the absence of a USP-NF Compounded Preparation Monograph on CNSP-Specific Stability Information
Is Covetrus Compounding Pharmacies USP compliant?
- Covetrus is compliant with USP standards
- Environmental and personnel monitoring occurs at our facilities
- We are compliant with USP 795, 797, and 800 standards that have come about in the past couple of years
What are we doing to address these changes and ensure continuity of care for your patients?
- Evaluation and testing to extend beyond use dating (BUD)
- For sterile compounds, we have an in-house lab so we do not have to send our products out for sterility testing, which expedites the process and allows more time for the end-user to use the product before BUD
- Expansion of our 503B catalog
- Smaller batch sizes made more frequently
- Prescriptions with smaller quantities and more frequent refills
- Utilizing bracket testing to cover more ranges of affected products
- Our Covetrus Prescription Management Platform allows veterinarians to easily update patient prescriptions to smaller order quantities when needed. Patients can also be set up on auto ship to receive those smaller quantities more frequently
Will I need to write all new prescriptions on November 1, 2023?
- No, new prescriptions will only be needed if we are making a change to the product, for example, switching from a water-based liquid to an oil-based preparation. All prescriptions written prior to November 1st will continue to be filled as written unless the pharmacy contacts the practice for notification of a change in the formulation of the product.
If I prescribe something with a beyond-use date that is shorter than the amount prescribed, what will happen?
- If a prescription is written past the expiration date of the product, the practice will be contacted by our pharmacy to alert them of the dating and adjust the quantity prescribed. Our pharmacy has quality checks in place to help ensure the product dating would support the days supply dispensed on the prescription.
Does USP apply to medications coming from a 503B facility for office use?
- No. Our 503B outsourcing facility, Atlas Pharmaceuticals, is not impacted by the updated USP 795 and 797 chapters as this pharmacy is regulated by cGMP standards.
When prescribing through the Covetrus Online Pharmacy, where can we see the current maximum BUD for each compounded product?
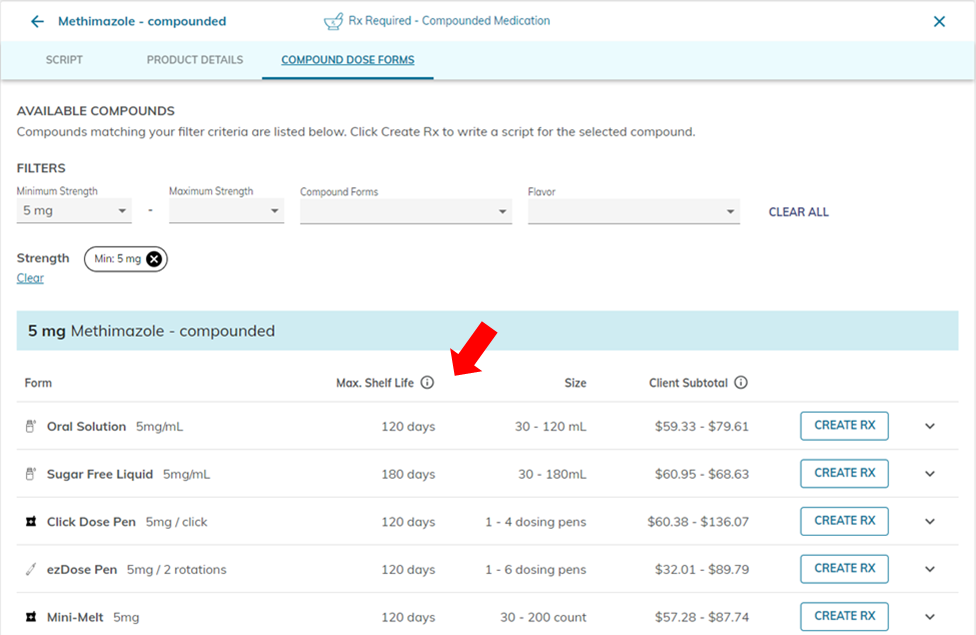
What is the best way to reach a pharmacist if we have questions?
- Call Covetrus® Compounding Pharmacies at (877) 518-4589
RESOURCES:
1. US Pharmacopeia (USP) – https://www.usp.org/ – Accessed 8/1/2023
2. USP Chapter 795 – https://www.usp.org/compounding/general-chapter-795 – Accessed 8/1/2023
3. USP Chapter 797 – https://www.usp.org/compounding/general-chapter-797 – Accessed 8/1/2023
4. <795>: Adding Flavor to Conventionally Manufactured Nonsterile Products – Accessed 11/1/2022
Call our pharmacy at (877) 518-4589 if you have any questions or if you would like to place a compounding order.
